The authors propose an evaluation method of textiles antiquity that could be applied to the Shroud. This paper was presented in the Symposium held by CIELT (Centre International d'Études sur le Linceul de Turin) in Rome - June 10th-12th 1993
Optical activity and racemization
Several organic substances, many of which of natural origin and biologically valid, present a fundamental property called optical activity.
Generally the light, and particularly sun light, is composed, as wave phenomenon, by many radiations that vibrate in every possible plane perpendicular to the propagation direction. The particular propagation-radiation that vibrates on a sole plane is called polarized.
The substances furnished with optical activity (optically active) are those able to turn to a certain angle, to the right or to the left, the vibration plane of the polarized light that passes through them.
The instrument that is able to measure such a deviation is called polarimeter. A polarimetric apparatus is formed by a monochromatic light lamp; to experiment with optically active substances it should be used a particularly pure light: the yellow light of a sodium lamp.
The light is directed through a polarizer (usually a Nicol prism), which serves as a first polaroid lens.
The light coming out the Nicol is polarized. The sample to be analysed is placed in solution in the analysing tube of the instrument after being chemically processed in an appropriate way. The possible changes is the substance to test are read and quantified.
In the polarimeter the sample to test, because of his composition, turns the light to a certain angle that the eye of the observer values on a graduated scale inside the apparatus.
The instrument therefore is formed by a rotation analyser (because the light passes through) with the survey scale of the angle; a rotated plane of the light; an analytical glass tube in which the solubilized sample to test is put; the polarized light plane; the polarizer and finally the sodium lamp. The instrument's optic observation point is on the rotation analyser side because from that point the light passes through.
The rotation angle that must be imparted to the right or to the left to the polarimeter's Nicol analyser charged with a substance to test optically active represents, in determinate experimental conditions (type of light, temperature of experiment, conditions of brightness), a fixed constant characteristic of the substance in examination.
The rotation angle (alpha) depends on quite a lot of factors: on the substance nature and on that of its solvent; on the quantity of the substance in solution passed through by the polarized light; on the wave length of the light the experience takes place with; on the temperature the test is realized to.
Experimentally determining the alpha angle for each active substance, when the substance is analyzed in solution, its specific rotation is calculated through the following equation:
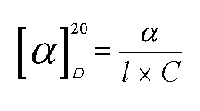
where alpha is the rotation angle measured through the polarimeter;
C is the concentration of the substance in examination expressed in mg/ ml;
l is the length in dm of the tube containing the sample;
20 indicates that the temperature is 20 degrees centigrade for the reading;
D reveals the wave length (showing the characteristic spectral line of the yellow sodium light).
The characteristic rotation for each optically active substance could result positive or negative according to the sign of the alpha rotation angle. This is assumed positive to show that the optically active substance is dextrorotatory and negative if the substance is laevorotatory.
After all, if the optically active substance turns the polarized light plane to the right is called dextrorotatory, otherwise it is laevorotatory or laevogyrous.
After discovering that certain organic substances are optically active, the phenomenon is explained by considering the molecular structure of the substance.
The start to investigate the cause of the rotatory power of a substance in its molecular structure was given by experimental tests. Samples of degraded sugars gave various results, compared to reference standards. The concept of racemization depends generally on a loss of optical activity in the investigated substance.
Racemes resolution in their optical opposites
Using optically active substances as starting reactives, one always note, at the end of each reaction, higher or lower quantities of racemes next to, vice versa, lower or higher quantities of one of the optical opposites. Furthermore, the same optical opposites present in the synthesis products tend, during the extraction and purification processes, to racemize.
In the chemical syntheses leading to asymmetrical compounds, one always obtains racemes due to reasons of equiprobability of the two configurations optically opposite formation.
The racemic modifications in liquid or gaseous state, or in solution, have identical properties (obviously in relation to a symmetrical reference) with those of the single optical opposites. Generally the racemization concept is connected with the substance optical activity loss and this is what happens when one is concerned with molecules with only an asymmetrical carbon atom or with molecular dissymetry. In molecules with more than an asymmetrical carbon atom there is racemization only when all the chiral centres undergo processes from an entropic point of view. One of them consists in using reactions leading to the reversible breakup of one of the asymmetrical carbon atom bonds.
A mixture in equal parts of two optical opposites constitutes a racemic modification that, obviously, will result optically inactive and it is indicated with the symbol (±) or D, 1 preceding the compound name. Examples: the tartaric or D, 1 tartaric acid is a mixture in equal parts of the isomers 45 and 46; if one adds the Grignard reactive to the acetic aldehyde, one obtains the secondary alcohol 53 which has an asymmetrical carbon atom:
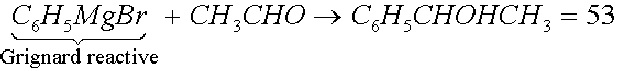
Racemic modifications in the liquid or gaseous state, or in solution, have similar properties referring to those of the single optical opposites and have the same infrared spectrum, a different refraction index and a different density. For the compounds with functional groups and not very reactive or without functional groups it could be achieved their resolution through the preparation of molecular or of inclusion complexes with reagents. It could be useful also the chromatography on dissymmetric absorbents (lactose) or on symmetrical absorbents (treated silica gel).
Resolution methods
The success of a racemic modification resolution is estimated determining the optical purity of the solution in examination.
Naturally if the substance separation happened in a complete way one should have that the two enantiomorphes will have an optical purity equivalent to 100%. A lower optical purity, for example 80%, will point out that the product is constituted for 80% by that enantiomorph and the remaining 20% is a racemic modification.
The method in examination refers to that one of determinating of the optical purity and controlling the specific rotatory power of the substance in examination. The optical purity is given by the ratio:
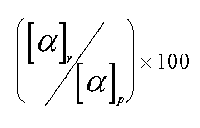
For the method application it is necessary to realize an acid hydrolysis of the sample to test through double normal hydrochloric acid at a temperature of 180 degrees centigrade for the minimum term of 120 minutes and a following filtering treatment on porous septum bukner. Natural fibers for the analysis are undressed and accurately weighed (up to milligram).
The acid containing solution depolymerizes the sugars with an inversion to hexoses, glucose and fructose. Dextrorotatory sugars separates from the laevorotatory ones. The value of deplacement (gradation) is measured by means of a polarimeter. The value obtained is the algebraic sum of all present sugars.
Experimental tests showed that optically active fibers can be separated, whereas ancient or very ancient fibres don't present optical activity, or they racemize.
The sample with controlled pH shall be examined with a polarimetric reading. The experimental phase was applied to samples of various historical periods that appeared on the average degraded according to their preservation state. The various readings through the polarimeter showed the characteristic (degradation or not) of the substance in examination.
For the experimental study it was applied the polarimeter COSMO Type-K 0032 (0-32%) with double scale - sugar scale 0-95% - ND.20 from 1.333 to 1.530. Precision 0.2% between 0 and 50%; 0.1% between 50 and 100%. Code WT-1; WT-4; WyF5090 with graduation of the scale.
In laboratory fibers from different ages were analyzed, between them Egyptian, Egyptian-copte, Roman and medieval ones.
A sample of optically active natural fibres that weighed 50 mg, analyzed in laboratory and historically dated back to the first Middle Age, showed a rotatory specific power of 41.2 degrees and of mg/ml density 0.861 in a 20 cm planimetric tube.
Another sample from Egyptian age, drawn from the bandaging of some mummies, appeared lacking in optical activity; while a sample dated back to the first century gave reference values higher than the Egyptian one but considerably lower than the medieval one. The specific rotatory power of this sample was 18.6 degrees.
The applied method, besides the "depolymerization" one, can therefore confirm integrity losses due to chemical decay of the fibers.
The results have been quite interesting and have pointed out morphological and structural degradation conditions, depending also on their preservation state.